The Food and Drug Administration (FDA) and the European Commission (EC) have made the traceability of medical devices (MD) a strategic priority
developing internationally harmonized legislation for the univocal identification of such equipment, named with the acronym UDI (Unique Device Identifier).
The FDA issued the standard on 24 September 2013. The full application is set on 24 September 2021, for class I and unclassified medical devices (later you can find the classification of MD), while for devices that require a permanent marking the date will be on 24 September 2022.
As regards the EU, two new regulations were issued in 2017:
- Regulation (EU) 2017/745 on medical devices;
- Regulation (EU) 2017/746 on in vitro diagnostic medical devices.
The full application of the two Community Regulations is set on 26 May 2021 for medical devices, while for medical-diagnostic devices in vitro will be on 26 May 2022.
Classification of medical devices
All manufacturers of medical devices, which fall within the classification drawn up according to the intended use and the degree of risk, must comply with UDI regulations. Specifically, the DMs are divided into four classes I, IIa, IIb, and III with a higher degree of risk of the device from class I to class III.

What is the UDI system?
The UDI code is made up of a series of characters, numeric or alphanumeric, created based on internationally accepted standards. It has as its purpose the unequivocal recognition of individual devices on the market. It must be obtained before placing the device on the market.
The UDI includes the UDI-DI and the UDI-PI.
UDI-DI (Device Identifier): it is a mandatory and fixed code, specific to a device model and is also used as an “access key” to the information stored in the UDI database;
UDI-PI (Production Identification): is a conditional and variable code, which includes: the serial number, the batch number, the expiry date, and the production date. This information is essential for the tracking and tracing of the device.
What is the Basic UDI-DI?
The Basic UDI-DI, whose acronym is BUDI-DI, identifies a group of devices with identical similar characteristics. For example devices with the same intended use, same risk class, and essential design and manufacturing characteristics. This code is not shown on the product label, but just in the technical documentation relating to the same. It is also the main key for registrations in the EUDAMED (EU) or GUDID (US) database.
What are the benefits of adopting the UDI system?
The implementation of the UDI system will ensure easy traceability of medical devices, improving the efficiency of the healthcare system supply chain. Specifically, UDI will allow the monitoring of medical devices along the entire chain, providing accurate and timely information to operators. Consequently, there will also be positive feedback on patient safety activities. The UDI could allow medical facilities to remove an unsafe product before it reaches the patient. Finally, the use of this system should also improve waste purchasing and disposal policies and stock management by health institutions and other economic operators.
To sum up, the UDI system is synonymous with:
- Increased protection of public health and patient safety;
- Rapid detection and more accurate monitoring of faulty device reports;
- Reduction of errors due to manual operations;
- Improvement of the after-sales analysis of medical devices placed on the market;
- Guarantee of a secure distribution chain, helping to fight counterfeiting and diversion;
- Development of a worldwide recognized medical device identification system.
Who issues the UDI code?
There are only four organizations recognized by the European Commission and the FDA for issuing UDI codes:
- GS1 AISBL;
- Health Industry Business Communications Council (HIBCC);
- International Council for Commonality in Blood Banking Automation (ICCBBA);
- Informationsstelle für Arzneispezialitäten (IFA).
Where should the UDI be affixed?
The labeling of the medical device and its packaging must show the complete UDI code (UDI-DI and UDI-PI). For each production, the code must be generated, printed, and affixed. The affixing of the code follows precise rules and does not replace other marking and labeling requirements.
The UDI should be represented on the medical device label in text format so that it can be easily read by people (HRI) and in AIDC format (e.g., a bar-code or an RFID tag), meaning any technology that transmits the unique device identifier, in a form which may be included in an electronic medical record or another computer system, using an automated process.
However, some peculiar situations are highlighted:
- Where important constraints are limiting the use of both AIDC and HRI formats on the label, only the AIDC format may appear;
- The UDI vector shall be reported in such a way that the AIDC is accessible during normal operating or storage conditions;
- Reusable devices shall carry a UDI vector on the device itself. The UDI vector of reusable devices that require cleaning, disinfection, sterilization, or refurbishment at intervals of use by the patient, shall be permanent and legible after each process designed to make the device ready for subsequent use, throughout the expected life of the device.
What to do in such cases?
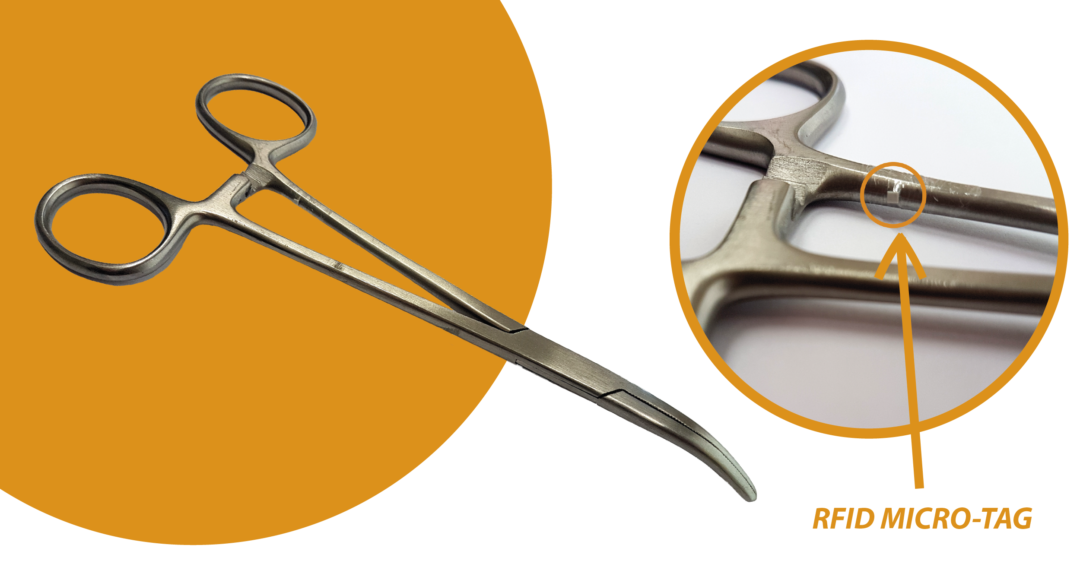
There are millimeter-sized RFID tags on the market. One of the companies that have invested most in these small transponders is the Japanese giant Murata, which has developed a series of micro-tags dedicated to healthcare sector, which can also be used on metal surfaces, resistant to thermal shocks and chemicals . They are therefore unalterable to the sterilization cycles in an autoclave and with gamma rays.
Given the following characteristics, ultra-small tags present significant advantages when compared with the most used bar codes and 2D codes. The latter in fact:
- Not suitable for small devices;
- Do not resist for a long time in difficult environments that can compromise reading;
- Shall not be incorporated into the device;
- They can be detached, damaged, or easily removed.
- They can only be read
On the contrary, the embeddable micro-tags:
- They are molded/embedded in the product, absolutely not interfering in terms of aesthetics and use;
- They cannot be removed or damaged;
- They last a long time being resistant to water and low temperatures;
- They are writable. The information contained within the single tag can be updated via software without physically intervening on the label.
If you want to know more about embedded micro-tags and their uses → click here
Due to the very small size, not all RFID readers can read these small transponders, especially if they are fixed on metal surfaces. For their effective reading, therefore, highly performing devices are required, equipped with optimized viewfinder antennas.
In the video below, we can see TERTIUM’s mobile RFID reader reading a Murata micro-tag, embedded in a needle holder. The RFID reader, supporting the NFC URI RTD and Text RTD data exchange formats, allows pointing to a specific web page, through the simple reading of the micro-tag, without the need to activate any app or software.
To conclude, in light of the benefits listed above, implementing an RFID system with micro-tags allows medical device manufacturers to meet the requirements of the UDI system, as well as providing a means to enable additional monitoring and inventory applications in the supply chain and healthcare facilities.
RFID is the right technology to take full advantage of the many opportunities offered by UDI data!!!
Contact us to know more → info@tertiumtechnology.it

